1. Introduction
Immunotherapy has attracted attention in recent years as the fourth treatment modality in cancer treatment, following surgery, anticancer drugs and radiotherapy. Immunity is a mechanism that recognizes and eliminates foreign substances and also contains a mechanism that controls excessive immune responses and protects the self. Immune checkpoint inhibitors such as PD-1 antibodies activate the immune system by releasing the immune system’s negative regulatory mechanisms, leading to cancer regression. However, immune checkpoint inhibitors are only effective in ~20% of solid tumors, and only in some cancer patients. The importance of CTL induction in cancer regression was reaffirmed.
On the other hand, peptide vaccine therapy, which identifies tumour-specific antigens and enhances antigen-specific CTLs with antigen peptides and adjuvants, has been attempted in vain. Induction of tumor-specific CTLs requires an adjuvant (priming adjuvant) that activates antigen-presenting dendritic cells together with the cancer antigen. In peptide vaccine therapy, inorganic substances such as aluminum hydroxide gel (Alum) and oils are used, but these adjuvants activate the immune system by inducing inflammation rather than activating dendritic cells, and are therefore inadequate for CTL induction. Development of priming adjuvants that can activate dendritic cells and induce cancer antigen-specific CTLs is needed.
2. Needs of Th1 (CTL-inducing) adjuvant
Within the dendritic cell subset, professional antigen-presenting dendritic cells (CD8α+ DCs in mice, CD103+ DCs in mice and CD141+ DCs in humans) have the ability to take up antigens from outside the cell and present them as 8˜9 mer peptides on MHC class I molecules to CD8+ T cells (cross-presentation). The activation of tumor-specific CTLs requires the presentation of cancer antigens by professional antigen-presenting dendritic cells, increased expression of co-stimulatory molecules (CD80, CD86) on dendritic cells and cytokines such as type I IFN and IL-12, which are expressed on dendritic cells in response to innate immune pattern recognition. TLR3 is highly expressed in endosomes of antigen-presenting dendritic cells, where it recognises double-stranded RNA (dsRNA), a virus-specific structure, and signals via TICAM-1 (also known as TRIF). TICAM-1 signaling activates IRF-3, NF-κB, IRF-1 and AP-1 transcription factors, induces cytokine production such as IFN-β and IL-12, increased expression of co-stimulatory molecules and provoke a pathway for antigen cross-presentation.
Poly(I:C), a synthetic dsRNA currently used as a TLR3 ligand, has potent adjuvant and anti-cancer activity, but systemic IFN-α/β and inflammatory cytokine production via MDA5, which is expressed in a wide range of cells, is a problematic because of adverse effects represented by cytokinemia. Therefore, the development of adjuvants that do not activate intracellular RIG-I-like receptors such as MDA5, but only TLR3, can reduce side effects by not inducing excessive inflammatory responses while maintaining anticancer activity. We attempted to reduce toxicity by modifying dsRNA derived from a measles virus vaccine strain (known as its safety) and finally developed ARNAX, a non-inflammatory adjuvant that is selective for dendritic cell TLR3 and induces CTL by attaching a DNA cap to 140-mer dsRNA (Matsumoto et al., Nat Commun. 2015) (Fig. 1).
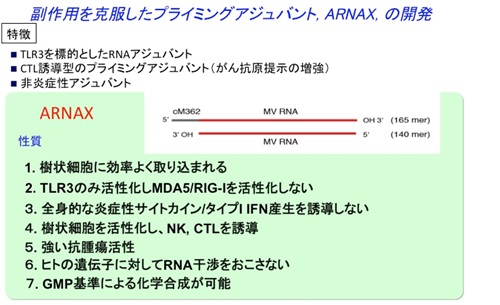
Figure 1. The properties of ARNAX
Vaccine therapy with ARNAX and cancer antigens reduced multiple types of cancer by inducing anti-cancer CTLs through the TLR3-TICAM-1-IRF3-IFN-β pathway in dendritic cells and infiltrating into the tumor without inducing systemic inflammatory cytokines in a mouse implant cancer model, and the effect of PD-L1 antibodies was enhanced, in some cases, into complete remission (Takeda et al., Cell Rep. 2017). The combination treatment consisting with ALNAX+PD-L1 antibodies enhanced Th1 polarization and CTL induction in the lymph nodes (priming phase), and the infiltration of CD8+ T cells in the tumor was also increased, resulting in proliferation and activation of antigen-specific CTLs. Blocking the PD-1/PD-L1 pathway accelerates CTL induction by ARNAX in dendritic cells in the priming phase, allowing more CTLs to infiltrate the tumor and effectively attack the tumor by disabling CTL suppression in the intra-tumor microenvironment (effector phase). ARNAX is the first non-inflammatory dendritic cell-targeting adjuvant that does not cause RNA interference with human genes, is safe due to the absence of cytokine toxicity and is potentially applicable to patients with a variety of solid tumors that do not remit with PD-1-only antibody therapy. The unmet needs for refractory cancers that do not respond to existing therapies are extremely high, and cancer vaccines containing ARNAX, a novel adjuvant that induces tumor antigen-specific CTLs, and combination therapy with PD-1/PD-L1 antibodies could be a promising treatment option in the future.
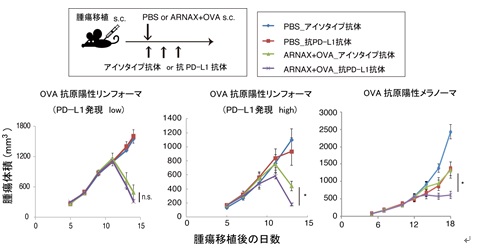
Figure 2. Anti-tumor effects of ARNAX+ antigen alone and in combination with anti-PD-L1 antibody Top figure: dosing protocol. Bottom figure: tumor regression by PD-L1 antibody alone, ARNAX+OVA alone or ARNAX+OVA in combination with PD-L1 antibody against PD-L1 low-expressing EG7 tumors (OVA-expressing murine lymphomas) (left figure), PD-L1 high-expressing EG7 tumors (middle figure) and OVA-expressing melanomas (right figure).
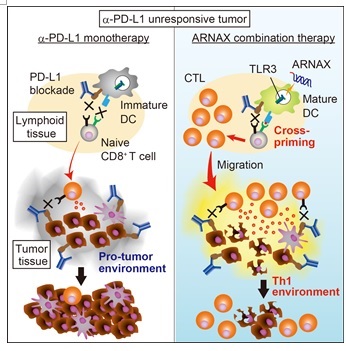
Figure 3. ARNAX therapy for cancers unresponsive to PD-1/PD-L1 antibodies (adapted from Cell Reports) Left: In cancers unresponsive to PD-1/PD-L1 antibodies, tumor-specific CTLs are low and the cancer proliferates. Right: ARNAX + cancer antigen administration induces tumor-specific CTLs, which infiltrate into the tumor and lead to tumor regression. When combined with anti-PD-L1 antibody, CTL induction is further enhanced in the lymph nodes of the tumour, leading to tumour regression.
3. Developmental policy in ARNAX
Priming adjuvant therapy is CTL induction by dendritic cell activation and targets the priming phase. When cancer antigens are released by cell death and are intrinsic, CTL induction can be achieved by adjuvant alone, but for stronger CTL induction, simultaneous administration with cancer antigens is necessary, and the selection of cancer antigens is important. Based on the knowledge of cancer antigens accumulated in the field of cancer vaccine, combination with natural peptides or proteins of cancer-specific antigens or cancer-related antigens CD4 and CD8 is considered to be effective. The integration of efficient RNA synthesis methods for preclinical and clinical trials and the search for biomarkers that can predict effective cases are important issues. ARNAX may modulate tumor microenvironment, which is an issue to be analyzed.
The laboratory plans to establish scale-up manufacturing methods and specification testing methods for ARNAX under GMP standards, select cancer antigens and obtain non-clinical POC for ARNAX alone and in combination with cancer antigens using transplanted mouse cancer models with high cancer antigen expression, and conduct non-clinical safety studies in a sequential manner.